State the number of valence electrons for a hydrogen atom and a chlorine atom. State the number of valence electrons for a sodium ion and a chloride ion in an ionic bond. State the number of valence electrons for a hydrogen atom and a chlorine atom in a covalent bond. Jan 31, 2021 The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of chlorine (3s²3p⁵). Thus, chlorine has seven valence electrons. When you refer to valence electrons, you only look at the last shell of the atom. In chlorine, the electron configuration is 2.8.7, thus chlorine has 7 valence electrons. How may electrons are in a. How many valence electrons are in these atoms: Potassium, Carbon, Magnesium, and Oxygen? Calcium-loses 2, Fluorine-gains 1, Aluminum-loses 3, Oxygen-gains 2 How many electrons will each of these elements gain or lose in forming an ion: calcium, fluorine, aluminum, and oxygen?
- How Many Valence Electrons Does Chlorine Have
- How Many Valence Electrons Are In Chlorine
- Number Of Valence Electrons In Chlorine Ion
- Cl Number Of Valence Electrons
- Valence Electrons Chart
A neutral atom has the same number of protons, neutrons, and .
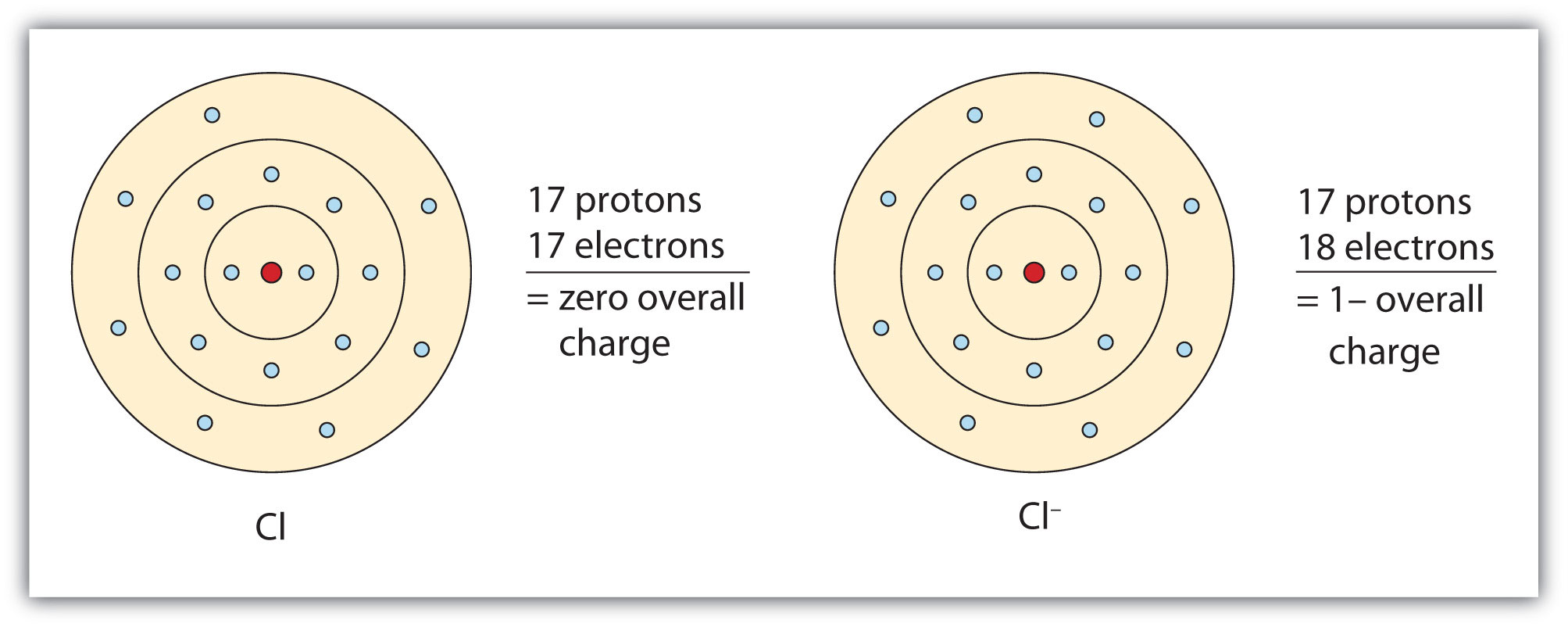
This diagram shows the electron shell configuration of a chlorine atom. Draw a Bohr Model of Chlorine (Cl) Atomic Number: 17 (# of protons & therefore, same # of electrons) Atomic Mass: (Atomic mass – Atomic number = # of.
[Bohr Model of Chlorine], Number of Energy Levels: 3. First Energy Level: 2. Second Energy Level: 8.
Third Energy Level: 7. [Bohr Model of Chlorine], Number of Energy Levels: 3.

First Energy Level: 2. Second Energy Level: 8. Third Energy Level: 7.
Similarly, neon has a complete outer 2n shell containing eight electrons. In contrast, chlorine and sodium have seven and one electrons in their.A Bohr diagram depicts an atom with a small, central nucleus and the electrons in their valence shells.
The first valence shell contains 2 electrons, and the second and third shell have 8 electrons each, and the number keeps growing. To draw the Bohr diagram for 'NaCl', we should first draw the individual diagrams for both 'Na' and 'Cl'.
The atomic number of 'Na' is 11, so it has 11 electrons. Bohr-Rutherford Diagrams ATOMS to IONS For each element: 1.
How Many Valence Electrons Does Chlorine Have
Write the standard atomic notation in the box. 2. Find the number of protons, neutrons, and electrons in each atom.
3. Draw the Bohr-Rutherford diagram.
4. Find the ion that this atom would create and write it in the box.
5. Draw the diagram for the ion created from the element. p n p+.
How Many Valence Electrons Are In Chlorine
Both elements have three electron shells. Sodium has one electron in its outer shell and chlorine has seven.
Neither of them has an outer shell that is filled, so these atoms are not very stable on their own. Facts Date of Discovery: Discoverer: Carl Wilhelm Scheele Name Origin: From the Greek word khlôros (green) Uses: Water purification, bleaches Obtained From: Salt Related Links Note: The external links below are not a part of this site and their content is not the responsibility of this site.
Number Of Valence Electrons In Chlorine Ion


Cl Number Of Valence Electrons
Valence Electrons Chart
lewis dot diagram for chlorine simple bright – elektronik what does sharing of electrons mean google electron dot formula electron dot formula for h2o lewis dot structure table big illustration great truncated 8 5 drawing lewis structures chemistry libretexts covalent bond lewis bonding theory sodium electron dot diagram gallery for sodium chloride 10 4 writing lewis structures chemistry.Bohr Diagram for Sodium and ChlorineChemical schematron.org - Chlorine (Cl)
